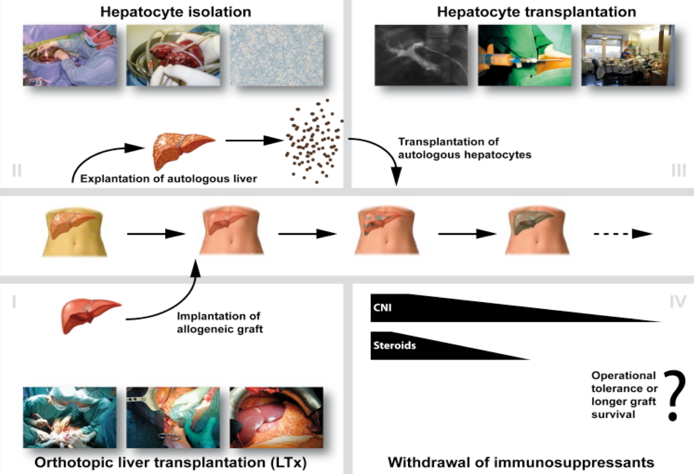
NeoHybrid Liver Graft
Once these cells have engrafted, it is postulated that the autologous cells will repopulate the allogeneic graft liver as they have a selective advantage over the donor tissue due to their autologous origin. It is hypothesized that this will lead to a neo-hybrid liver graft, reducing the need for immunosuppression and possibly inducing tolerance to both the entire engrafted tissue and the allogeneic donor matrix.
Aim of the project is to show proof of principle for this concept in a rat model, combined with the establishment of isolation procedures and characterisation of hepatic progenitors and adult liver cells from diseased human livers obtained during orthotopic LTx.
neoHybrid Liver Graft
Once these cells have engrafted, it is assumed that autologous cells will repopulate the allogeneic liver, since they should have a selective advantage due to their autologous origin. It is postulated that this will lead to a neo-hybrid liver graft, reducing immunogenicity and inducing immunoregulation thus minimizing the need for extensive immunosuppression and eventually inducing operational tolerance.
In order to investigate the feasibility of this new approach, studies in animal models as well as investigations on human livers explanted during standard orthotopic LTx are essential. Animal models for isolation and induction of hepatocyte and “oval cell” proliferation are well established and their positive effect in cell transplantation has already been proven experimentally. In the rat model, detailed studies on liver repopulation by transplanted cells can be realized using retrorsine for selectively blocking the liver cell cycle of the recipient.
The tolerogenic potential of transferred autologous liver cells will be investigated in a rat liver transplant model. In this model, post-transplant immunosuppressive therapy will be based on calcineurin inhibitors (CNI). Immunosuppressive protocols based on CNI are clinically used for LTx and seem appropriate for liver cell transplant studies since these substances do not perturb transplanted hepatocytes. The effects of weaning of immunosuppression will be investigated in this small animal model. Additionally, the tolerogenic potential of autologous liver cells will be further challenged by generating a pre-transplant donor-reactive memory in the recipient, as memory T and B cells represent a major barrier for long-term graft function in clinical transplantation.
In parallel, the efficiency of large-scale cell isolation from diseased human livers explanted during LTx will be investigated. The isolated cells will be cultivated and characterised in order to enable a specific prediction of the fractions of cells that can be achieved from diseased organs under conditions suitable for clinical application. In order to evaluate the repopulative capacity, these cells will be analysed in an immunodeficient small animal model. The studies on isolation of cells from diseased human livers will build the basis for further clinical translation of the neohybrid liver graft concept.