Liver Cell Transplantation
Liver cell transplantation (LCT) is a promising approach for treating certain liver diseases like metabolic disorders as well as chronic or acute liver failure. LCT is based on the infusion of liver cells; it is therefore less invasive than OLT and can be performed repeatedly if required. Clinical trials of LCT have shown promising results for the treatment of liver-based metabolic disorders, but the clinical success of LCT still remains limited, especially for treatment of acute or chronic liver failure.
Several barriers to the successful treatment of liver disease by LCT have been identified, in particular the insufficient long-term engraftment and function of transplanted cells in the liver. Methods for non-invasive monitoring of liver cell transplantation are not available for clinical routine. Workable extrahepatic sites for liver cell implantation are not clinically established which would be desirable for treatment chronic liver failure, in which the deranged liver architecture might prevent hepatocyte engraftment.
The lab has focused on two major aspects in further development of LCT: the monitoring of LCT using Magnetic Resonance Imaging and the evaluation of routes for administration of liver cells.
Several barriers to the successful treatment of liver disease by LCT have been identified, in particular the insufficient long-term engraftment and function of transplanted cells in the liver. Methods for non-invasive monitoring of liver cell transplantation are not available for clinical routine. Workable extrahepatic sites for liver cell implantation are not clinically established which would be desirable for treatment chronic liver failure, in which the deranged liver architecture might prevent hepatocyte engraftment.
The lab has focused on two major aspects in further development of LCT: the monitoring of LCT using Magnetic Resonance Imaging and the evaluation of routes for administration of liver cells.
Monitoring of liver cell transplantation with MRI
MRI enables the non-invasive assessment and visualisation of anatomy with very high spatial resolution and excellent soft tissue contrast. Compared to other non-invasive visualisation strategies such as computer tomography or scintigraphy, MRI requires no gamma- or x-ray exposition. Therefore, this method provides advanced safety to the patient, enables real-time and repetitive examinations as well as intra-operative cell tracking. Cells can be visualized by MRI following labeling with superparamagnetic iron oxide particles as intracellular contrast agent. Several types of iron oxide particles are available, from ultrasmall to micron-sized iron oxide particles.
Feasibility of MRI monitoring of human liver cells: Initial results with Tat-peptide modified MagForce particles
As first attempt to investigate the feasibility of monitoring human liver cells with MRI, nano-sized, superparamagnetic MagForce particles were used. Cells were investigated via a clinical MR scanner at 3.0 Tesla and the particle uptake within single hepatocytes was estimated using fluorescence microscopy. The labelled primary human hepatocytes were clearly detectable by MRI, proving the feasibility of this new concept. However, cells were not detectable on a single cell level.
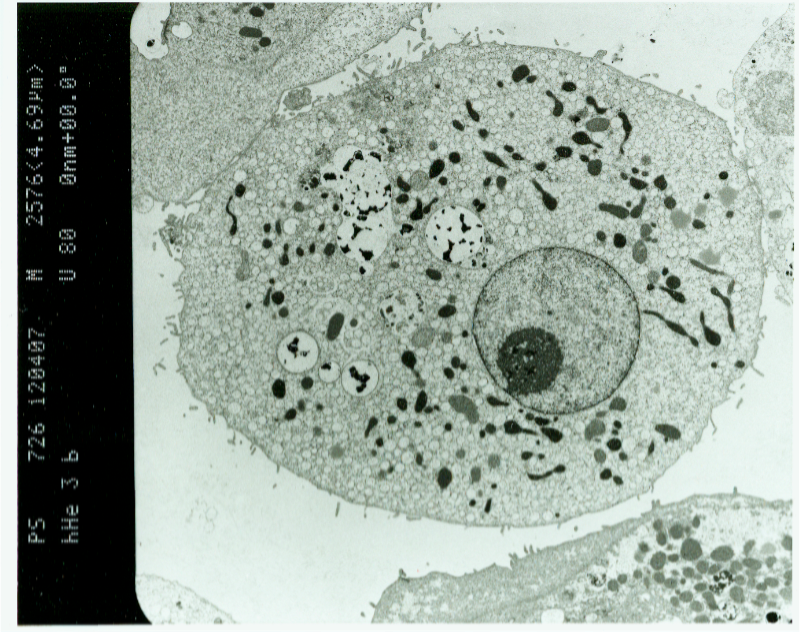
Labeling of human liver cells with MPIO enables in vitro detection of liver cells on a single cell level
In order to achieve fast labeling, high safety of the particle load and the detectability of labeled cells on a single cell level, micron sized iron oxide particles (MPIO) were introduced to cellular imaging and Shaprio et al. showed, that MPIO-labeled hepatocytes could be detected on a single cell level in a murine model using 7,0 and 11,0 Tesla MRI. Based on these results, we investigated the feasibility of labeling of human liver cells with MPIO. We showed that human hepatocytes could be sufficiently labeled with MPIO. MPOI-labeled primary human hepatocytes were detectable by clinical MR equipment in vitro on a single cell level. MPIO-labeling had no adverse effects on viability or metabolic activity of labeled human liver cells. Thus, we provided a protocol for labeling of human liver cells with MPIO that could be used for further preclinical and clinical studies.
For more detailed information please refer to J Cell Mol Med. 2008 Aug;12(4):1384-94.
For more detailed information please refer to J Cell Mol Med. 2008 Aug;12(4):1384-94.
Comparison of MPIO and SPIO for labeling of human liver cells
The clinically approved dextran-coated SPIO are biodegradable, whereas polymer-embedded MPIO, which are not clinical grade, are supposedly bio-inert. Several studies have shown that neither type of particle affects the cellular viability or metabolic activity of labeled cells. For MPIO, which have not been investigated to that extent, concerns remain with respect to their large iron core. In order to further investigate the safety of MPIO-labeling of human hepatocytes, we compared the effects of both types of particles on ROS formation, cellular integrity, and synthetic activity of labeled. Incubation of human liver cells with both types of particles enabled particle uptake, but labeling with MPIO was successful within a dramatically shorter incubation time (4 hours vs. 16 hours). The biodegradable SPIO caused temporary ROS formation and non-physiologic activation of the iron metabolic pathway, suggesting intracellular iron release that exceeded clearance by the usual mechanisms of cellular iron metabolism. MPIO labeling did not cause ROS formation or perturbation of the cellular iron homeostasis. Thus, we concluded that MPIO-labeling is safe, whereas SPIO-labeling could have adverse effects on human liver cells.
For more detailed information please refer to Mol Imaging Biol. 2011 Aug;13(4):613-22.
For more detailed information please refer to Mol Imaging Biol. 2011 Aug;13(4):613-22.
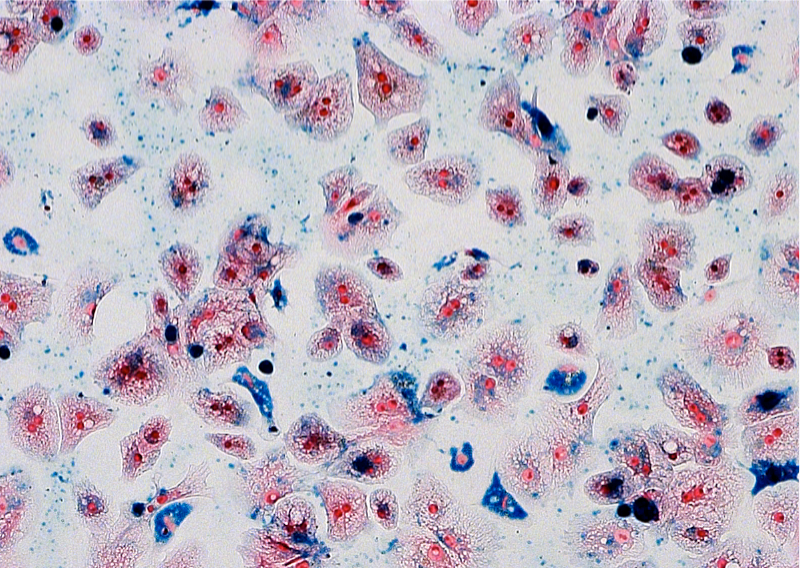
Labeling of primary human hepatocytes with MPIO in suspension culture
For clinical applications, large numbers of cells must be labeled in a simple and rapid manner and have to be applied in suspension. However, all existing protocols are based on adhesion culture labeling with subsequent resuspension, only suitable for small experimental settings. We investigated the feasibility of preparing MPIO- labeled primary human hepatocytes in a temporary suspension culture using the Rotary Cell Culture System (RCCS). We were able to provide PHHs labeled for detection with clinical MRI equipment within 4 h after isolation ready for application. The labeling was stable during the investigation period of 6 days and no obvious adverse effects were detected. The results gained from this study may further be transferable for labeling of other types of adherent-dependent cells with MRI contrast agents. With no additional transfection agents, scaffolds, or digestion enzymes being used, the protocol may be used for large-scale GMP-conforming processing for clinical application in the future.
For more detailed information please refer to Artif Organs. 2011 Apr;35(4): 91-100.
Preclinical studies on MRI monitoring of LCT
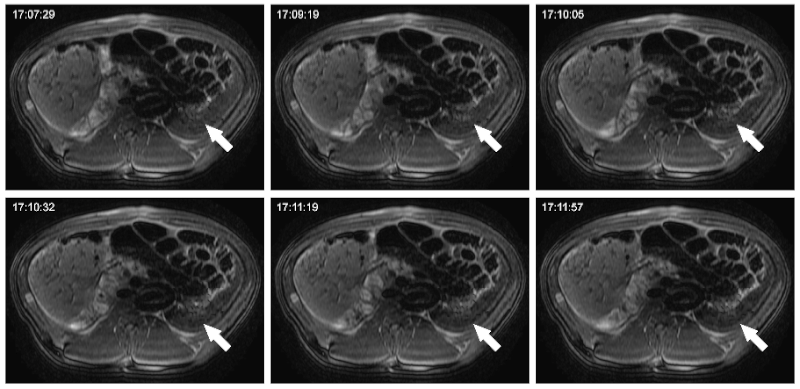
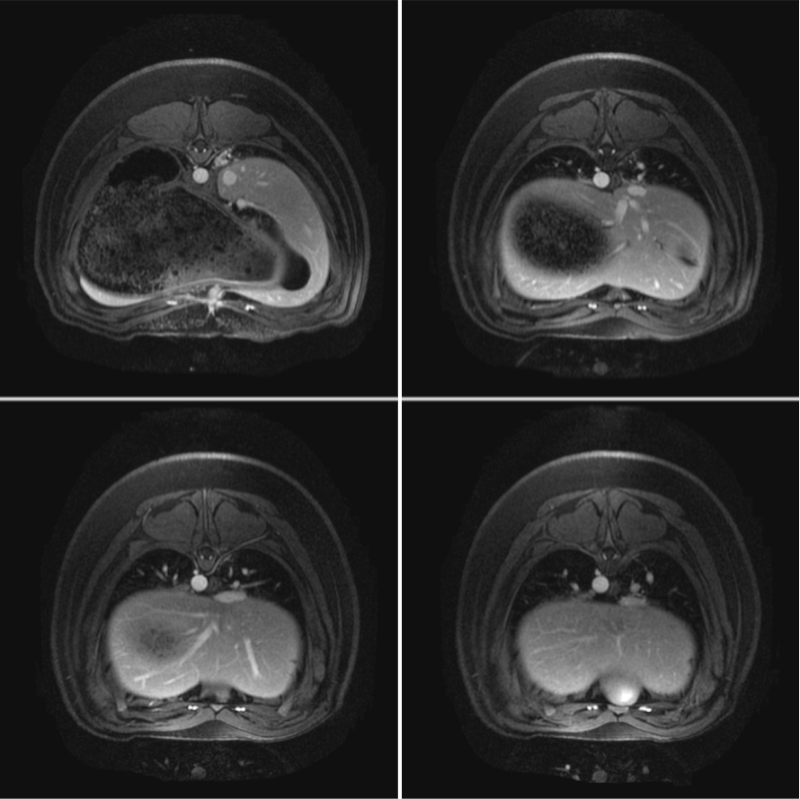
Studies on cellular imaging with MRI are usually performed in vitro or in vivo. Despite the scientific benefit from these studies, the results of these studies cannot be directly conferred to the clinics since clinically applicable MRI equipment and imaging protocols often not provide comparable image resolution and sensitivity. In order to investigate the feasibility of MRI monitoring of liver cells under preclinical conditions, we performed imaging studies in a preclinical swine model. Initially, a threshold for detectability of labeled porcine liver cells using abdominal 3.0 Tesla MRI was defined. Allogeneic liver cells were then transplanted into the liver or spleen and animals were investigated by repeated MRI and CT up to 8 weeks after transplantation. Following intraportal application of MPIO-labeled liver cells, signal voids were detected in the liver of the LCT recipients. These signal voids were identified to be caused by MPIO-labeled liver cells that had microembolized in distal portal branches. Microembolization is not an uncommon event in clinical intraportal LCT, but conventional imaging technologies are not adequate for their visualization. Thus, we showed that MRI following transplantation of MPIO-labeled liver cells is suited to visualize these microembolizations. When cells engrafted without microthrombi formation in this model, we did not find signal voids in the liver. Thus, our study showed that MRI can be a valuable tool for noninvasive elucidation of cellular processes of LCT under clinical conditions. MRI can be used to visualize the microembolization of transplanted liver cells following intraportal infusion, undetectable with conventional noninvasive imaging modalities.
For more detailed information please refer to Cell Med. 2010 1(3):123–135 and Cell Med. 2010 1(3)113-114
Evaluation nof different routes of application and extrahepatic implantation sites for LCT
Administration of liver cells via the portal vein is so far the route most commonly utilized in clinical LCT. However, intraportal LCT is associated with the risk of microthrombi formation. Therefor, alternative routes for LCT have to be investigated. Moreover, workable extrahepatic sites for liver cell implantation are not clinically established which would be desirable for treatment chronic liver failure, in which the deranged liver architecture might prevent hepatocyte engraftment. The spleen was previously considered as the most appropriate alternative implantation site, and the splenic route for LCT has already been investigated in clinical trials that have shown some improvement of clinical parameters after splenic LCT.
We investigated three different routes of administration for liver cells in a preclinical swine model: Intraportal infusion into the liver, direct injection into the splenic parenchyma, and intra-arterial infusion to the spleen. Intraportal LCT caused significant microthrombi formation in the distal branches of the portal system. Cells directly injected into the spleen were retained and temporary engrafted in the spleen, whereas cell infusions intra-arterially into the spleen led to translocation and engraftment of transplanted cells in the liver, with significantly fewer microembolisms compared to intraportal application. Thus, the results of this study suggest that the intra-arterial route deserves further clinical evaluation, with a special focus on cell engraftment within the liver rather than hepatization of the spleen.
For more detailed information please refer to Cell Med. 2010 1(3):123–135
