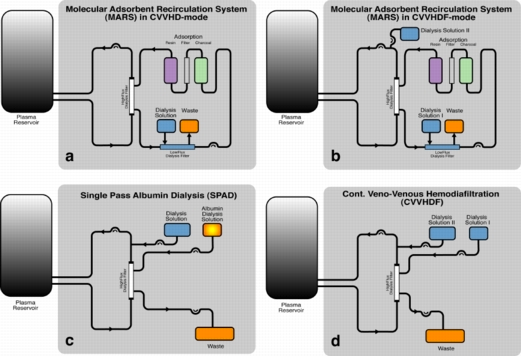
Artificial Liver Support
With the growing disparity between the numbers of suitable donor organs and patients waiting for transplantation, efforts have been made to optimize the allocation of organs, to find alternatives to cadaveric liver transplantation and to develop extracorporeal methods to support or replace the failing organ. Moreover, in the case of hepatic failure, patients with a capacity for liver recovery could be bridged to regeneration and would not require transplantation at all. In cases of chronic liver failure, temporary extracorporeal liver support therapy may be beneficial in episodes of acute deterioration (acute-on-chronic liver failure). The accumulation of water-soluble toxins (e.g. ammonia, mercaptans) and of a number of albumin-bound water insoluble toxins of pathogenetic relevance in liver failure (e.g. bilirubin, bile acids, short chain fatty acids, and aromatic amino acids) has been implicated as a cause of hepatic encephalopathy and organ dysfunction in patients with liver failure.
Conventional continuous veno-venous hemodiafiltration (CVVHDF) has been shown to be effective in the removal of water-soluble toxins. In order to clear the blood of albumin-bound, hydrophobic substances, additional adsorber or acceptor substances are necessary to enhance mass exchange. Albumin is one of the potential acceptor substances. Stange, Mitzner and co-workers introduced a detoxification system based on albumin dialysis - the Molecular Adsorbents Recirculation System (MARS). Separated from the patient’s blood by a high-flux hemodialysis filter, an albumin solution is circulated in a closed circuit. The albumin acts as the acceptor for the toxins and is partly regenerated by passing an anion exchanger and a charcoal adsorber in a closed circuit and is itself dialyzed by a standard dialysis solution in continuous veno-venous hemodialysis (CVVHD) or CVVHDF operation mode. In order to operate the system, additional hardware (MARS monitor) including a recirculation pump is necessary.
Conventional continuous veno-venous hemodiafiltration (CVVHDF) has been shown to be effective in the removal of water-soluble toxins. In order to clear the blood of albumin-bound, hydrophobic substances, additional adsorber or acceptor substances are necessary to enhance mass exchange. Albumin is one of the potential acceptor substances. Stange, Mitzner and co-workers introduced a detoxification system based on albumin dialysis - the Molecular Adsorbents Recirculation System (MARS). Separated from the patient’s blood by a high-flux hemodialysis filter, an albumin solution is circulated in a closed circuit. The albumin acts as the acceptor for the toxins and is partly regenerated by passing an anion exchanger and a charcoal adsorber in a closed circuit and is itself dialyzed by a standard dialysis solution in continuous veno-venous hemodialysis (CVVHD) or CVVHDF operation mode. In order to operate the system, additional hardware (MARS monitor) including a recirculation pump is necessary.
Single Pass Albumin Dialysis
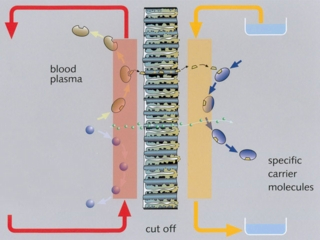
Single-pass albumin dialysis (SPAD) is a simple method of albumin dialysis using standard renal replacement therapy machines without an additional perfusion pump system: The patient’s blood flows through a circuit with a high-flux hollow fiber hemodiafilter, identical to that used in the MARS system. The other side of this membrane is cleansed with an albumin solution in counter-directional flow, which is discarded after passing the filter. CVVHDF can be performed in the first circuit via the same high-flux hollow fibers. Waiving of sorbent columns for regeneration of the adsorber-albumin, initial clinical application of SPAD was performed with higher concentrations of albumin in the dialysis fluid than in the MARS concept.
Single pass albumin dialysis (SPAD), the Molecular Adsorbents Recirculation System (MARS) and continuous veno-venous hemodiafiltration (CVVHDF) were compared in vitro with regard to detoxification capacity.
In each experiment 4100 ml of toxin-loaded human plasma was processed for 6.5 hours. MARS treatment (n=6) was performed in combination with CVVHDF. For SPAD (n=6) and CVVHDF (n=6) a high-flux hollow fiber hemodiafilter (identical to the MARS filter) was used. Levels of ammonia, urea, creatinine, bilirubin, and bile acids were determined. Concentrations before and after application were calculated as differences and compared by the Kruskal-Wallis test. Post hoc comparisons for testing pairs of groups were adjusted according to Bonferroni-Holm. Time, group and interaction effects were tested by using the non-parametric ANOVA model for repeated measurements according to Brunner et al. .
SPAD and CVVHDF showed a significantly greater reduction of ammonia compared with MARS. No significant differences could be observed between SPAD, MARS and CVVHDF concerning other water-soluble substances. SPAD enabled a significantly greater bilirubin reduction than MARS. Concerning the reduction of bile acids no significant differences between SPAD and MARS were seen. When operating MARS in CVVHD mode as recommended by the manufacturer, no significant differences were seen concerning the removal of bilirubin, bile acids, urea, and creatinine. However, MARS in CVVHD mode was significantly less efficient in removing ammonia than in CVVHDF mode.
In each experiment 4100 ml of toxin-loaded human plasma was processed for 6.5 hours. MARS treatment (n=6) was performed in combination with CVVHDF. For SPAD (n=6) and CVVHDF (n=6) a high-flux hollow fiber hemodiafilter (identical to the MARS filter) was used. Levels of ammonia, urea, creatinine, bilirubin, and bile acids were determined. Concentrations before and after application were calculated as differences and compared by the Kruskal-Wallis test. Post hoc comparisons for testing pairs of groups were adjusted according to Bonferroni-Holm. Time, group and interaction effects were tested by using the non-parametric ANOVA model for repeated measurements according to Brunner et al. .
SPAD and CVVHDF showed a significantly greater reduction of ammonia compared with MARS. No significant differences could be observed between SPAD, MARS and CVVHDF concerning other water-soluble substances. SPAD enabled a significantly greater bilirubin reduction than MARS. Concerning the reduction of bile acids no significant differences between SPAD and MARS were seen. When operating MARS in CVVHD mode as recommended by the manufacturer, no significant differences were seen concerning the removal of bilirubin, bile acids, urea, and creatinine. However, MARS in CVVHD mode was significantly less efficient in removing ammonia than in CVVHDF mode.

In vitro test setup for evaluation of detoxification techniques
The detoxification capacity of SPAD was shown to be similar or even higher when compared with MARS. As the SPAD setup is less complex and less expensive, the method is currently under clinical evaluation at our centre.
In our in vitro set-up we used a 4.4 % solution of human albumin. This concentration is the one we use in our clinical setup, based on case reports using this technique for extracorporeal detoxification 11, and on previous in vitro studies by Awad et al. As albumin is the most expensive component in albumin dialysis, further dose-determining studies must be performed: the ideal concentration and flow rate of the albumin-solution has to be identified. These in vitro studies are currently underway.
For more detailed information please refer to the following publications:Hepatology 2004, 39: 1408-1414 and Artificial Organs 2002; 26: 703-706.
In our in vitro set-up we used a 4.4 % solution of human albumin. This concentration is the one we use in our clinical setup, based on case reports using this technique for extracorporeal detoxification 11, and on previous in vitro studies by Awad et al. As albumin is the most expensive component in albumin dialysis, further dose-determining studies must be performed: the ideal concentration and flow rate of the albumin-solution has to be identified. These in vitro studies are currently underway.
For more detailed information please refer to the following publications:Hepatology 2004, 39: 1408-1414 and Artificial Organs 2002; 26: 703-706.
Development of an artificial liver support system
Conventional continuous veno-venous hemodiafiltration is effective in the removal of water-soluble toxins only. In order to clear the blood of albumin-bound, hydrophobic substances, additional adsorber or acceptor substances are necessary to enhance mass exchange.
Aim is the development of an efficient and economic artificial liver support concept based on the transmembranous elimination of toxins relevant in liver failure with alternative synthetic substances – the nanosorbents. In a disposable ad-on for standard renal replacement therapy units, the patient’s blood flows through a circuit with an optimised hollow fiber hemodiafilter. The other side of this membrane is cleansed with a nanosorbent solution in counter-directional flow, which is discarded after passing the filter. A screening platform (1:30 and 1:125 in vitro model) has been developed for evaluating nanosorbents considering the complex dynamic interactions of adsorber substances, dialysis fluid dynamics, and hollow fiber specifications with human blood or plasma.
Aim is the development of an efficient and economic artificial liver support concept based on the transmembranous elimination of toxins relevant in liver failure with alternative synthetic substances – the nanosorbents. In a disposable ad-on for standard renal replacement therapy units, the patient’s blood flows through a circuit with an optimised hollow fiber hemodiafilter. The other side of this membrane is cleansed with a nanosorbent solution in counter-directional flow, which is discarded after passing the filter. A screening platform (1:30 and 1:125 in vitro model) has been developed for evaluating nanosorbents considering the complex dynamic interactions of adsorber substances, dialysis fluid dynamics, and hollow fiber specifications with human blood or plasma.