Bioartificial Liver Support
Acute liver failure (ALF) has a poor prognosis with mortality rates between 50% and 90% under conservative management. Over the last 50 years, advances have been made in the understanding of the pathophysiology encompassing the clinical features of ALF: encephalopathy, cerebral edema, hemorrhage, electrolyte and metabolic disturbances, renal failure, cardiovascular instability and increased risk of infection.
While cerebral edema is the most common cause of death, multi-organ failure and sepsis are also associated with significant mortality. Depending on etiology, the survival rate in acute liver failure under conservative treatment ranges from 79% (amanita intoxication) to 10% (cryptogeneic genesis). Introduction of liver transplantation (LTx) as therapeutic option reduced mortality to 20 - 40%.
With the continued, growing disparity between the numbers of organ donations and patients waiting for liver transplantation, efforts have been made to optimize the allocation of organs and to design extracorporeal methods to support or replace the failing liver. The frequent lack of donor livers for urgent transplantation in ALF highlights the need for a liver support therapy until an organ becomes available. Moreover, patients with a capacity for liver recovery could be bridged to regeneration and would not require transplantation at all.
An extracorporeal liver support system has to support or substitute the main functions of the liver, providing detoxification, synthesis and regulation. To date, developments concentrate on either artificial detoxification systems (such as albumin dialysis or adsorber suspension with activated charcoal particles and ion exchange resin), or on biologic systems (like whole organ perfusion and bioreactors with liver cells).
For detoxification (e.g. removal of bilirubin, bile acids, and toxins) simple, dialysis-like artificial detoxification systems have been shown to be efficient. The complex tasks of regulation (e.g. CNS transmitter precursors) and synthesis (e.g. coagulation factors) remain to be addressed by the use of human liver cells.
The Modular Extracorporeal Liver Support (MELS) is an integrative concept for the treatment of hepatic failure with appropriate extracorporeal therapy units, tailored to suit the actual clinical needs of the patient. The CellModule is a bioreactor charged with primary human liver cells, harvested from human donor livers found to be unsuitable for transplantation due to steatosis, cirrhosis or traumatic injury. The DetoxModule enables albumin-dialysis for the removal of albumin-bound toxins, reducing the biochemical burden of the liver cells, and replacing the bile excretion of hepatocytes in the bioreactor. Continuous veno-venous hemofiltration can be added to the system if required.
For more detailed information please refer to the following publications:
Hepatology 2004, 39: 1408-1414
Artificial Organs 2005, 29: 141-151
Transplantation 2003, 76: 781-786
Journal of Hepatology 2003, 39: 649-653
Journal of Clinical Virology 2003, 28: 141-154
Xenotransplantation 2003, 10: 460-469
International Journal of Artificial Organs 2002, 25: 1001-1005
Artificial Organs 2002; 26: 703-706 and
Xenotransplantation 2002; 9: 309-324.
While cerebral edema is the most common cause of death, multi-organ failure and sepsis are also associated with significant mortality. Depending on etiology, the survival rate in acute liver failure under conservative treatment ranges from 79% (amanita intoxication) to 10% (cryptogeneic genesis). Introduction of liver transplantation (LTx) as therapeutic option reduced mortality to 20 - 40%.
With the continued, growing disparity between the numbers of organ donations and patients waiting for liver transplantation, efforts have been made to optimize the allocation of organs and to design extracorporeal methods to support or replace the failing liver. The frequent lack of donor livers for urgent transplantation in ALF highlights the need for a liver support therapy until an organ becomes available. Moreover, patients with a capacity for liver recovery could be bridged to regeneration and would not require transplantation at all.
An extracorporeal liver support system has to support or substitute the main functions of the liver, providing detoxification, synthesis and regulation. To date, developments concentrate on either artificial detoxification systems (such as albumin dialysis or adsorber suspension with activated charcoal particles and ion exchange resin), or on biologic systems (like whole organ perfusion and bioreactors with liver cells).
For detoxification (e.g. removal of bilirubin, bile acids, and toxins) simple, dialysis-like artificial detoxification systems have been shown to be efficient. The complex tasks of regulation (e.g. CNS transmitter precursors) and synthesis (e.g. coagulation factors) remain to be addressed by the use of human liver cells.
The Modular Extracorporeal Liver Support (MELS) is an integrative concept for the treatment of hepatic failure with appropriate extracorporeal therapy units, tailored to suit the actual clinical needs of the patient. The CellModule is a bioreactor charged with primary human liver cells, harvested from human donor livers found to be unsuitable for transplantation due to steatosis, cirrhosis or traumatic injury. The DetoxModule enables albumin-dialysis for the removal of albumin-bound toxins, reducing the biochemical burden of the liver cells, and replacing the bile excretion of hepatocytes in the bioreactor. Continuous veno-venous hemofiltration can be added to the system if required.
For more detailed information please refer to the following publications:
Hepatology 2004, 39: 1408-1414
Artificial Organs 2005, 29: 141-151
Transplantation 2003, 76: 781-786
Journal of Hepatology 2003, 39: 649-653
Journal of Clinical Virology 2003, 28: 141-154
Xenotransplantation 2003, 10: 460-469
International Journal of Artificial Organs 2002, 25: 1001-1005
Artificial Organs 2002; 26: 703-706 and
Xenotransplantation 2002; 9: 309-324.
Modular Extracoporeal Liver Support
The liver support system consists of a blood circuit with a continuous plasma separation unit (e.g. Multifiltrate, Fresenius Medical Care AG, Bad Homburg, Germany), a high-flux dialysis filter (Fresenius Medical Care), and a second circuit for plasma perfusion of the bioreactor. The simple set-up allows the integration of any other standard renal replacement therapy machine.
Venous access is gained by placing a double-lumen dialysis catheter into either the internal jugular or the femoral vein. Blood is pumped through a hollow fiber plasma filter (e.g. Plasmaselect 0.4, Braun) at a rate of 150-250 mL/min. If necessary, a continuous infusion of heparin has to be performed for anticoagulation in order to achieve an activated clotting time of 160-180 seconds (e.g. ACT, Fresenius, Oberursel, Germany).
For continuous exchange with the CellModule the bioreactor is connected to the plasma circuit in counter-directional flow mode at 150-200 mL/min. The total extracorporeal volume is approximately 110 ml in the blood circuit, and 900 ml of plasma in the bioreactor and associated circuitry.
Venous access is gained by placing a double-lumen dialysis catheter into either the internal jugular or the femoral vein. Blood is pumped through a hollow fiber plasma filter (e.g. Plasmaselect 0.4, Braun) at a rate of 150-250 mL/min. If necessary, a continuous infusion of heparin has to be performed for anticoagulation in order to achieve an activated clotting time of 160-180 seconds (e.g. ACT, Fresenius, Oberursel, Germany).
For continuous exchange with the CellModule the bioreactor is connected to the plasma circuit in counter-directional flow mode at 150-200 mL/min. The total extracorporeal volume is approximately 110 ml in the blood circuit, and 900 ml of plasma in the bioreactor and associated circuitry.
MELS CellModule
The CellModule is a bioreactor charged with liver cells - e.g. the AMC-BAL available from R.A.F.M. Chamuleau and the company HepArt or the BR0600 multi-compartment bioreactor developed by J.C. Gerlach and provided by the company Hybrid Organ. In the latter a independent but interwoven hollow fibre bundles serve three functions: medium inflow, cell oxygenation/carbon dioxide removal, as well as medium outflow. Thus, decentralized plasma exchange and gas supply can be provided.
Polyether-sulphone (PES) hollow fiber membranes with a molecular weight cut-off of > 400,000 and a total surface of 2.11 square-meters are used for mass exchange and hydrophobic multi-laminate hollow fiber membrane systems with a total surface of 2.22 square-meters enable gas supply.
The interwoven hollow fibers form a three-dimensional capillary network with numerous subunits for decentralized high performance mass exchange for nutrient and substrate supply, as well as metabolite removal and cell aggregate immobilization:
two PES capillary systems are used for counter-directional flow perfusion of the cells with the patient’s plasma and one MHF capillary system enables integral oxygenation and carbon dioxide removal.
The interwoven small capillary subunits represent the artificial counterpart of natural liver lobuli. The extracapillary space forms a cell compartment in which the inoculated liver cells and hepatocyte nursing cells reside.
Polyether-sulphone (PES) hollow fiber membranes with a molecular weight cut-off of > 400,000 and a total surface of 2.11 square-meters are used for mass exchange and hydrophobic multi-laminate hollow fiber membrane systems with a total surface of 2.22 square-meters enable gas supply.
The interwoven hollow fibers form a three-dimensional capillary network with numerous subunits for decentralized high performance mass exchange for nutrient and substrate supply, as well as metabolite removal and cell aggregate immobilization:
two PES capillary systems are used for counter-directional flow perfusion of the cells with the patient’s plasma and one MHF capillary system enables integral oxygenation and carbon dioxide removal.
The interwoven small capillary subunits represent the artificial counterpart of natural liver lobuli. The extracapillary space forms a cell compartment in which the inoculated liver cells and hepatocyte nursing cells reside.
The CellModule is loaded with human liver cells, harvested from organs explanted for LTx but subsequently discarded due to steatosis, cirrhosis or mechanical injury. Cells are obtained by 5-step collagenase liver perfusion. Under conditions of capillary perfusion, parenchymal and non-parenchymal cells form tissue by spontaneous organization to cellular aggregates, immobilized to the surface of the capillaries. Within these aggregates, channels are formed, representing neo-sinusoidal structures with a reformation of a neo-space of Dissé. The cells produce their own biomatrix - the use of foetal calf serum and additional (animal derived) biomatrix collagen can be avoided. A cell mass of. 400g - 600g enables the clinical application of a liver lobe equivalent hybrid organ.
During stand-by, the cultures are continuously perfused with medium at a flow rate of 250 ml/min. Fresh medium is added continuously to the circuit at 150 ml/hour up to the third culture day, afterwards the feeding rate is reduced to 50 ml/h for the further culture period. Partial pressure of oxygen and carbon dioxide, and acid/base status were regularly measured by blood gas analysis.The cells produce their own biomatrix - the use of fetal calf serum and additional (animal derived) biomatrix collagen can be avoided. A cell-mass of 400 g – 600 g enables the clinical application of a liver lobe equivalent hybrid organ. During the stand-by phase of 21 days (mean) prior to therapeutic use, the bioreactors are characterized concerning individual metabolic activity on a daily basis and contamination can be excluded. This phase enables recovery of the cells, tissue-reformation, quality control, and clinical application on demand without cryopreservation.
During stand-by, the cultures are continuously perfused with medium at a flow rate of 250 ml/min. Fresh medium is added continuously to the circuit at 150 ml/hour up to the third culture day, afterwards the feeding rate is reduced to 50 ml/h for the further culture period. Partial pressure of oxygen and carbon dioxide, and acid/base status were regularly measured by blood gas analysis.The cells produce their own biomatrix - the use of fetal calf serum and additional (animal derived) biomatrix collagen can be avoided. A cell-mass of 400 g – 600 g enables the clinical application of a liver lobe equivalent hybrid organ. During the stand-by phase of 21 days (mean) prior to therapeutic use, the bioreactors are characterized concerning individual metabolic activity on a daily basis and contamination can be excluded. This phase enables recovery of the cells, tissue-reformation, quality control, and clinical application on demand without cryopreservation.
MELS DetoxModule
In liver failure the insufficient metabolism of endogenous toxins has been shown to be fatal. Most of these toxins are albumin-bound. In previous reports, liver assist devices based on albumin-dialysis were found to eliminate these toxins. , The DetoxModule enables albumin-dialysis via a standard high-flux dialysis cartridge. Single-pass albumin dialysis (SPAD) is a simple implementation of albumin dialysis using standard renal replacement therapy machines:
The patient’s blood flows through a circuit with a high-flux hollow fiber hemodiafilter (Fresenius HdF 100S polysulfone high-flux haemodiafilter, Fresenius AG, Bad Homburg). The other side of this membrane is cleansed by an albumin solution in counter-directional flow and discarded after passing the filter. One liter of a 4.5-liter bag with standard bicarbonate buffered dialysis solution is replaced by 1000 ml of 20% human albumin solution, resulting in 4.4% albumin solution. During therapy, the blood pump speed is adjusted to 130 –180 ml/min; the dialysis-pump speed is 600 ml/h.
30-75% of all patients in ALF show renal failure with fluid overload, electrolyte derangement and high levels of creatinin. Therefore, the MELS concept enables an integrated renal replacement therapy in terms of a continuous veno-venous hemodiafiltration via the high-flux hollow fiber hemodiafilter as part of the DetoxModule. A standard buffered aqueous solution is used (added after the filter, “postdilution”) with a flow rate of 1000 - 3000 ml/h.
Single pass albumin dialysis (SPAD) has been subject to extensive studies and comparison with other available systems like the Molecular Adsorbent Recirculations System (MARS), Prometheus and renal replacement therapy.
The patient’s blood flows through a circuit with a high-flux hollow fiber hemodiafilter (Fresenius HdF 100S polysulfone high-flux haemodiafilter, Fresenius AG, Bad Homburg). The other side of this membrane is cleansed by an albumin solution in counter-directional flow and discarded after passing the filter. One liter of a 4.5-liter bag with standard bicarbonate buffered dialysis solution is replaced by 1000 ml of 20% human albumin solution, resulting in 4.4% albumin solution. During therapy, the blood pump speed is adjusted to 130 –180 ml/min; the dialysis-pump speed is 600 ml/h.
30-75% of all patients in ALF show renal failure with fluid overload, electrolyte derangement and high levels of creatinin. Therefore, the MELS concept enables an integrated renal replacement therapy in terms of a continuous veno-venous hemodiafiltration via the high-flux hollow fiber hemodiafilter as part of the DetoxModule. A standard buffered aqueous solution is used (added after the filter, “postdilution”) with a flow rate of 1000 - 3000 ml/h.
Single pass albumin dialysis (SPAD) has been subject to extensive studies and comparison with other available systems like the Molecular Adsorbent Recirculations System (MARS), Prometheus and renal replacement therapy.
Clinical Application
Whether or not liver support devices are beneficial for the patient is rather controversial. An extracorporeal liver support system has to support or substitute the main functions of the liver, providing detoxification (e.g. toxins, ammonia, bilirubin, endotoxins), synthesis (e.g. albumin, amino acids, coagulation factors) and regulation (e.g. acid-base-status, electrolytes, amino acids, energy supply and transmitter precursors for the central nervous system). To date, scientific efforts concentrate on either artificial detoxification systems (such as albumin dialysis, adsorber suspension with activated charcoal particles and ion exchange resin) or on biological systems (bioreactors with liver cell cultures).
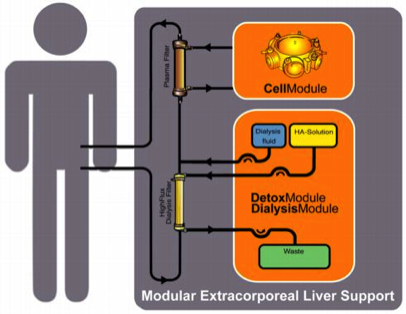
The modular extracorporeal liver support concept MELS combines different extracorporeal therapy units and enables synergistic use of available technologies. The application mode can be tailored to suit the individual needs of the patient and may be intensified according to the clinical course. A detoxification module enables single-pass albumin dialysis, a simple implementation of albumin dialysis using standard renal replacement therapy machines, and the use of a standard high-flux dialysis cartridge.
The DetoxModule enables removal of toxins from the patient’s blood, as well as reduction of the biochemical burden for the cells inside the bioreactor. In addition, it removes the bile produced by the cells inside the bioreactor. 30 to 75% of all patients in ALF show renal failure with fluid overload, electrolyte derangement and uremia.
Continuous veno-venuous hemodiafiltration, provided by the DialysisModule, permits detoxification and reduction of fluid overload. The understanding, that a critical issue of the clinical syndrome is the accumulation of toxins, not cleared by the failing liver, led to the development of artificial filtration and adsorbtion devices. The absence of certain hepatocyte-derived factors and lack of regulation is addressed by efforts to provide cell functions with hepatocyte-based systems.
The DetoxModule enables removal of toxins from the patient’s blood, as well as reduction of the biochemical burden for the cells inside the bioreactor. In addition, it removes the bile produced by the cells inside the bioreactor. 30 to 75% of all patients in ALF show renal failure with fluid overload, electrolyte derangement and uremia.
Continuous veno-venuous hemodiafiltration, provided by the DialysisModule, permits detoxification and reduction of fluid overload. The understanding, that a critical issue of the clinical syndrome is the accumulation of toxins, not cleared by the failing liver, led to the development of artificial filtration and adsorbtion devices. The absence of certain hepatocyte-derived factors and lack of regulation is addressed by efforts to provide cell functions with hepatocyte-based systems.
Phase I study with primary porcine liver cells
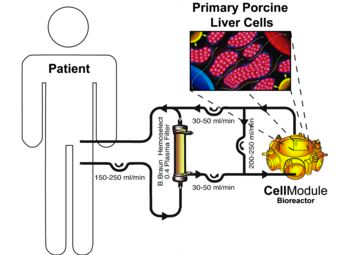
The objective of this study was to evaluate the feasibility and safety of a hybrid liver support system with extracorporeal plasmaseparation and bioreactor perfusion in patients with acute liver failure (ALF) who had already fulfilled the criteria for high-urgency liver transplantation.
8 patients (1 male, 7 female) were treated in terms of bridging to transplantation. The mean age was 36.5 years (range 20-58). Etiology of liver failure was drug-related (n=2), hepatitis B infection (n=3), and unknown for three patients. The bioreactors were charged with primary liver cells from specific pathogen-free pigs. Cell viability varied between 91 and 98%.
Continuous liver support treatment over a period of 8-46 hours (mean 27.3 h) was safely performed and well tolerated by all patients. No complications associated with the therapy were observed during the follow-up period. Thrombocytopenia was considered to be an effect of the plasmaseparation. Subsequently, all patients were transplanted successfully and were observed over at least two years with an organ and patient survival rate of 100%. Screening of patient’s sera for antibodies specific for porcine endogenous retroviruses (PERVs) showed no reactivity - either prior to application of the system, or after extracorporeal system.
The results encourage us to continue the development of the technology, and further studies appear to be justified. The bioreactor-technology has been integrated into a modular extracorporeal liver support (MELS) system, combining biologic liver support with artificial detoxification technology.
For more detailed information please refer toXenotransplantation 2003, 10: 460-469, Journal of Clinical Virology 2003, 28: 141-154 and Xenotransplantation 2002; 9: 309-324 .
8 patients (1 male, 7 female) were treated in terms of bridging to transplantation. The mean age was 36.5 years (range 20-58). Etiology of liver failure was drug-related (n=2), hepatitis B infection (n=3), and unknown for three patients. The bioreactors were charged with primary liver cells from specific pathogen-free pigs. Cell viability varied between 91 and 98%.
Continuous liver support treatment over a period of 8-46 hours (mean 27.3 h) was safely performed and well tolerated by all patients. No complications associated with the therapy were observed during the follow-up period. Thrombocytopenia was considered to be an effect of the plasmaseparation. Subsequently, all patients were transplanted successfully and were observed over at least two years with an organ and patient survival rate of 100%. Screening of patient’s sera for antibodies specific for porcine endogenous retroviruses (PERVs) showed no reactivity - either prior to application of the system, or after extracorporeal system.
The results encourage us to continue the development of the technology, and further studies appear to be justified. The bioreactor-technology has been integrated into a modular extracorporeal liver support (MELS) system, combining biologic liver support with artificial detoxification technology.
For more detailed information please refer toXenotransplantation 2003, 10: 460-469, Journal of Clinical Virology 2003, 28: 141-154 and Xenotransplantation 2002; 9: 309-324 .
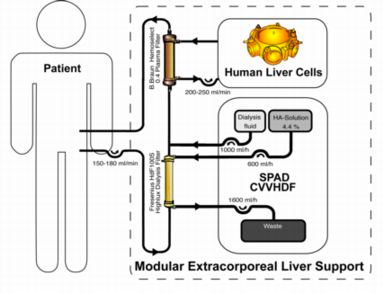
Phase I study with primary human liver cells
The patients are treated with the modular extracorporeal liver support concept. A bioreactor is charged with human liver cells, obtained from discarded cadaveric grafts, and integrated into an extracorporeal circuit with continuous single pass albumin dialysis and continuous veno-venuous hemodiafiltration for detoxification and fluid reduction.
Venous access is gained by placing a double-lumen dialysis catheter into either the internal jugular or the femoral vein. Blood is pumped through a hollow fiber plasma filter (Plasmaselect 0.4, Braun) at a rate of 150-250 mL/min. If necessary, a continuous infusion of heparin has to be performed for anticoagulation in order to achieve an activated clotting time of 160-180 seconds (ACT, Fresenius, Oberursel, Germany). For continuous exchange with the CellModule the bioreactor is connected to the plasma circuit in counter-directional flow mode at 150-200 mL/min. The total extracorporeal volume is approximately 110 ml in the blood circuit, and 900 ml of plasma in the bioreactor and associated circuitry.
The DetoxModule enables albumin-dialysis via a standard high-flux dialysis cartridge. The patient’s blood flows through a circuit with a high-flux hollow fiber hemodiafilter (Fresenius HdF 100S polysulfone high-flux haemodiafilter, Fresenius AG, Bad Homburg). The other side of this membrane is cleansed by an albumin solution in counter-directional flow and discarded after passing the filter. One liter of a 4.5-liter bag with standard bicarbonate buffered dialysis solution is replaced by 1000 ml of 20% human albumin solution, resulting in 4.4% albumin solution. During therapy, the blood pump speed is adjusted to 130 –180 ml/min; the dialysis-pump speed is 600 ml/h.
12 Patients with acute liver failure (n=3), primary non-function after LTx (PNF, n=3) and acute-on-chronic liver failure (AoCLF, n=6) were treated.
Venous access is gained by placing a double-lumen dialysis catheter into either the internal jugular or the femoral vein. Blood is pumped through a hollow fiber plasma filter (Plasmaselect 0.4, Braun) at a rate of 150-250 mL/min. If necessary, a continuous infusion of heparin has to be performed for anticoagulation in order to achieve an activated clotting time of 160-180 seconds (ACT, Fresenius, Oberursel, Germany). For continuous exchange with the CellModule the bioreactor is connected to the plasma circuit in counter-directional flow mode at 150-200 mL/min. The total extracorporeal volume is approximately 110 ml in the blood circuit, and 900 ml of plasma in the bioreactor and associated circuitry.
The DetoxModule enables albumin-dialysis via a standard high-flux dialysis cartridge. The patient’s blood flows through a circuit with a high-flux hollow fiber hemodiafilter (Fresenius HdF 100S polysulfone high-flux haemodiafilter, Fresenius AG, Bad Homburg). The other side of this membrane is cleansed by an albumin solution in counter-directional flow and discarded after passing the filter. One liter of a 4.5-liter bag with standard bicarbonate buffered dialysis solution is replaced by 1000 ml of 20% human albumin solution, resulting in 4.4% albumin solution. During therapy, the blood pump speed is adjusted to 130 –180 ml/min; the dialysis-pump speed is 600 ml/h.
12 Patients with acute liver failure (n=3), primary non-function after LTx (PNF, n=3) and acute-on-chronic liver failure (AoCLF, n=6) were treated.
