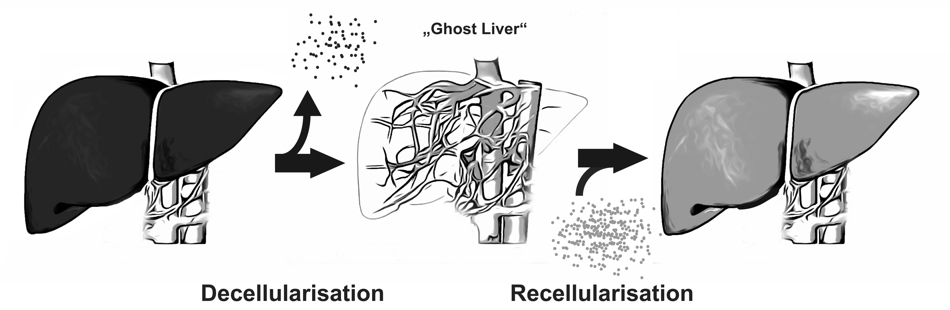
A new approach to overcome organ scarcity by in vitro generation of organoids is decellularisation and recellularisation of tissues or whole organs. This concept is based on the idea of composing biologic scaffolds by removing cellular and antigen-presenting components from tissues or organs, obtaining the organs’ extracellular matrix
A new approach to overcome organ scarcity by in vitro generation of organoids is decellularisation and recellularisation of tissues or whole organs. This concept is based on the idea of composing biologic scaffolds by removing cellular and antigen-presenting components from tissues or organs, obtaining the organs’ extracellular matrix (ECM).
The ECM represents the secreted products of resident cells and preserves the complex architecture and three-dimensional structure of the organ and thus generates a biomatrix ideally suited for reseeding with cells. This implies several advantages compared to other bioengineering concepts:
By preserving the organ’s ultrastructure, including the vascular network, oxygen and nutrient transport to the cells is warranted.
The ECM interacts with the reseeded cells and can act as a proliferation and differentiation inductive template for (autologous) stem cells or progenitor cells.
With this technique, scaffolds from marginal organs e.g. from non-heart beating donors or xenogeneic origin could be used for recellularization with the patient’s own cells. In this manner organ shortage could be overcome and post-transplant lifelong immunosuppressive therapy regimens would be dispensable. Another interesting potential application for these in vitro engineered organoids is their usage in pre-clinical drug discovery or toxicology or as a model for divers liver diseases (i.e. viral infections, hereditary metabolic diseases).
Recent reports on liver de- and recellularization provide evidence that the perfusion of alkaline detergents and/or enzymatic solutions via the portal vein is feasible to obtain a three-dimensional non-immunogenic biomatrix, that supports and improves survival and functionality of liver and/or progenitor cells. To enhance engraftment, migration and proliferation as well as to prevent dedifferentiation and loss of function of hepatocytes used for the repopulation of decellularized scaffolds, an adequate composition of micro-environmental conditions of the ECM is essential.
Since all known agents for liver decellularization cause alteration and damage to the ECM, a ‘gentler’ decellularization method may provide optimal support for cellular repopulation. On the other hand, ineffective decellularization leading to cellular and antigenic remnants within the ECM of allogenic or xenogenic origin could cause deleterious immunogenic effects (i.e., rejection) after implantation in vivo. Thus, finding an optimal compromise between cellular removal and preservation of the ECM composition (i.e., invasiveness of decellularization) should be addressed in further investigations to provide standardized decellularization protocols for livers from different species.
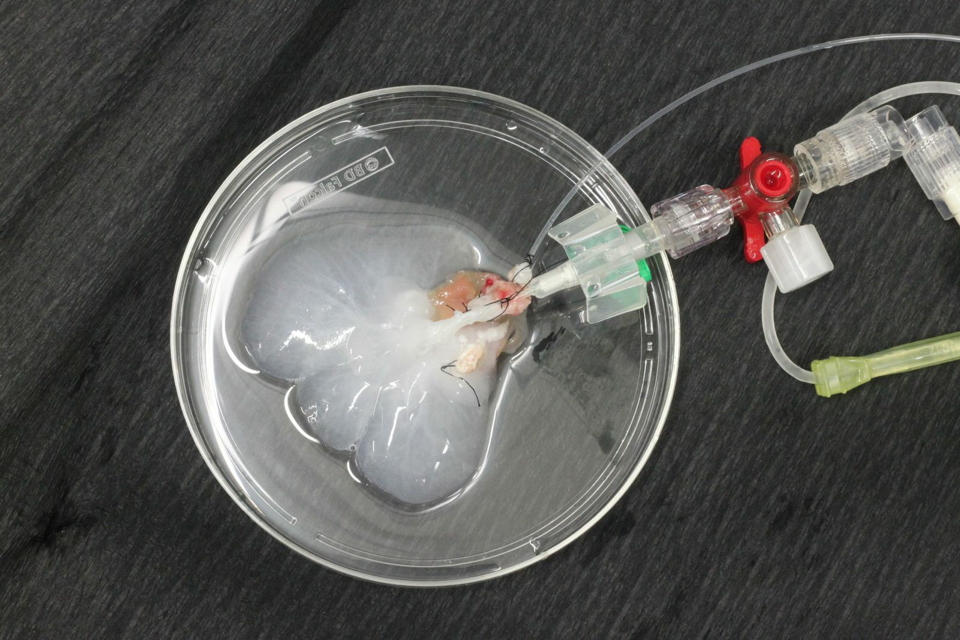
We developed a novel decellularization technique by perfusing with Triton X-100 and SDS via the hepatic artery under oscillating pressure conditions in a proprietary decellularization device. This device is designed to generate pressure changes on the perfused organ by mimicking intraabdominal conditions. In situ, the liver is adherent to the diaphragm and ‘hangs’ in its copula; the liver is affected mechanically by movement of the diaphragm during respiration. Whereas exhaling leads to lower intraabdominal pressure and increased portal-venous flow, inhaling (or increasing the intraabdominal pressure) causes higher outflow via the hepatic veins into the vena cava. This mechanism leads to a physiological aspiration of portal venous blood during expiration and a ‘squeezing’ of venous blood during inspiration, which can be likened to a ‘sponge’ and results in optimal perfusion of the liver.
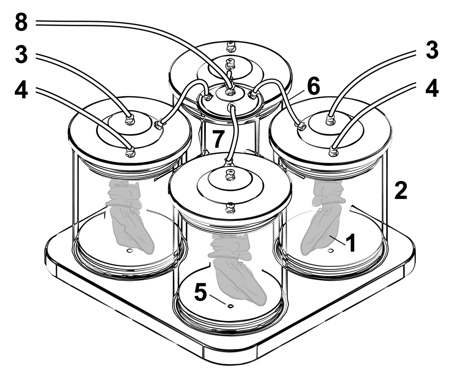
Nami R1.0 perfusion device enabling oscillating pressure conditions
(1) Perfused rat liver ‘hanging’ inside the sealed perfusion chamber (2) that is filled with sterile 0,9% NaCL solution (2). Tubes (3) for the selective perfusion of the portal vein and the hepatic artery (4). Luer-Lock-connectors are available for waste removal (5). A tube (6) connects the pressure distributor (7) with the perfusion chambers (2). A further tube (8) connects the pressure distributor /7) to the respirator (not shown).
The fact that changes in intraabdominal pressure and the movement of the diaphragm during respiration could affect liver perfusion has been previously mentioned, and the benefit of oscillating pressure conditions on extracorporeally perfused pig livers was confirmed experimentally by Neuhaus et al.. It was shown that oscillating pressure conditions improve the microperfusion within these organs and homogenize perfusions under physiological perfusion rates. Homogeneity of extracorporeal perfusion is important because cells in poorly perfused areas are ischemic and are more likely to perish. During perfusion decellularization, cells in poorly perfused areas do not come in contact with detergents and remain within the organ. Thus, to dissolve these remaining cells, either the perfusion rate (or pressure) can be increased or the perfusion can be extended.
Perfusion via the hepatic artery improves decellularization outcomes of rat livers compared to ‘conventional’ portal venous perfusion. Applying oscillating pressure conditions on extracorporeally perfused rat livers further enhances microperfusion and thus the homogeneity of perfusion decellularization. Using this technique the invasiveness of applied methodology can be reduced, resulting in a gentle and quick (3h) decellularization protocol for rat livers.
For more detailed information please refer to J Tissue Eng Regen Med. 2014:
Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions.Nami R2.0 is an improved rat liver perfusion device enabling oscillating pressure conditions (more details published soon)
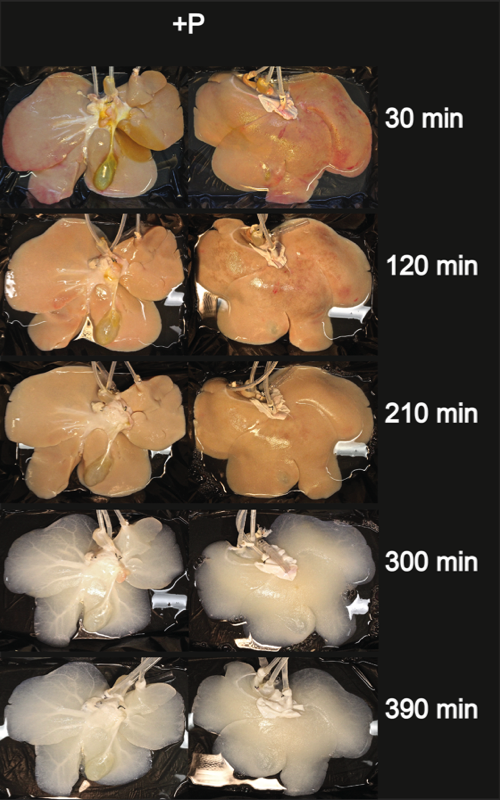
We developed a protocol for fast and homogenous porcne liver decellularization by simultaneous, pressure-controlled perfusion of detergents (i.e., 1% Triton X-100 and 1% SDS) via the portal vein and the hepatic artery. Livers were perfused under oscillating pressure conditions to improve the homogeneity and increase the effectiveness of the decellularization process. The oscillating pressure was generated by a vane-type pump, which improves microperfusion within livers by mimicking intraabdominal conditions during respiration. In situ, the liver is located under the copula of the diaphragm and follows the movement of the diaphragm during respiration. Thus, due to the lowering of the diaphragm and the increase in the intraabdominal pressure during inspiration, the liver is squeezed to optimize outflow of blood into the liver veins. During expiration, the diaphragm elevates the liver, the intraabdominal pressure decreases, and the inflow of portal venous blood into the liver is subsequently increased 20.
Our perfusion device mimics physiological, intra-abdominal conditions by generating slow oscillating pressures paralleling resting respiratory rates in humans. The effects of this device have previously been evaluated in extracorporeally perfused pig livers in in vitro and pre-clinical transplant models. Neuhaus, who developed this perfusion model, showed that oscillating pressure changes interfered reciprocally with portal venous and arterial perfusion flow (an effect that was confirmed in our experiments; data not shown) and significantly improved the homogeneity of liver perfusion.
He observed less cell damage and improved functionality in livers perfused for 24 hours under oscillating pressure conditions compared to livers perfused without oscillating pressure. Furthermore, he showed that the application of this device as an extracorporeal liver support improved the survival of hepatectomized pigs. Schoen et al. confirmed these results by using a modified version of the Neuhaus perfusion chamber. In his experiments, livers perfused under oscillating pressure conditions showed less endothelial cell damage compared to cold-stored livers. Furthermore, in an orthotopic transplant model, pigs showed improved survival if livers (warm ischemic time of 1h) were perfused for four consecutive hours under oscillating pressure conditions prior to implantation as compared to four hours of cold storage only. Schoen proposed that his normothermic extracorporeal liver perfusion model could serve as a conservation method for livers of non-heartbeating donors.
Two experimental groups were investigated, and livers were decellularized with (P+) or without (P-) the application of sinusoidal pressure changes to verify whether the application of oscillating pressure conditions improves the effectiveness of pig liver decellularization.
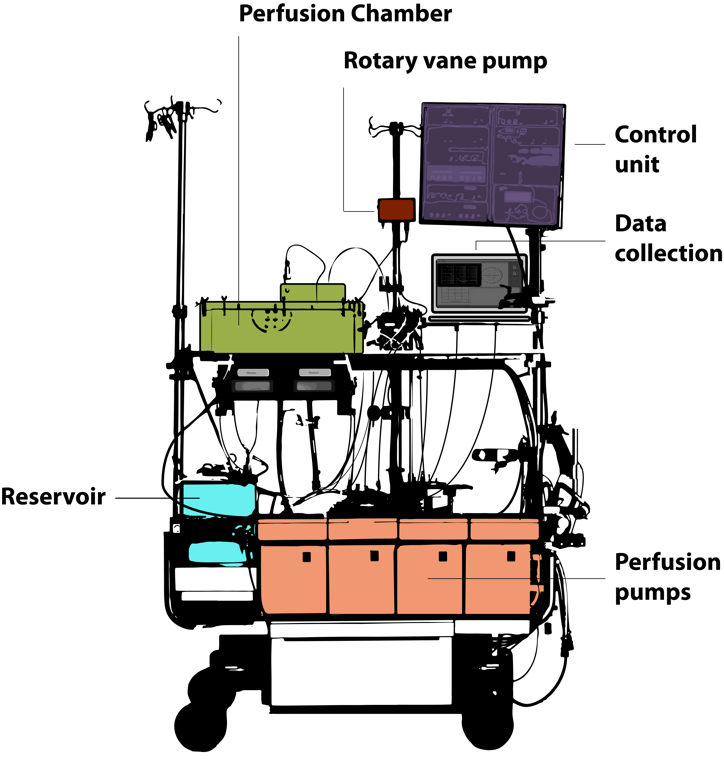
All perfusions were performed using a clinical-scale perfusion device. Depending on the liver size (and weight), perfusion rates via the portal vein ranged from 210 ml/min to 1240 ml/min and from 90 ml/min to 380 ml/min via the hepatic artery. However, the flow rates did not significantly differ in the two experimental groups neither at the beginning of perfusion (at 0 h) nor at the end of the decellularization process (after 6 h of perfusion with detergents). During decellularization, the flow rates slightly increased, although the differences were not significant in any of the groups.
The presented protocol for pig liver decellularization is quick (7 h) and effective. The application of oscillating pressure conditions improves the homogeneity of perfusion and thus the outcome of the decellularization process. The presented decellularized liver matrices could serve as scaffolds for organoid generation on a clinically relevant scale. Furthermore, an adapted version of our perfusion settings may be applied for the decellularization of discarded human livers.