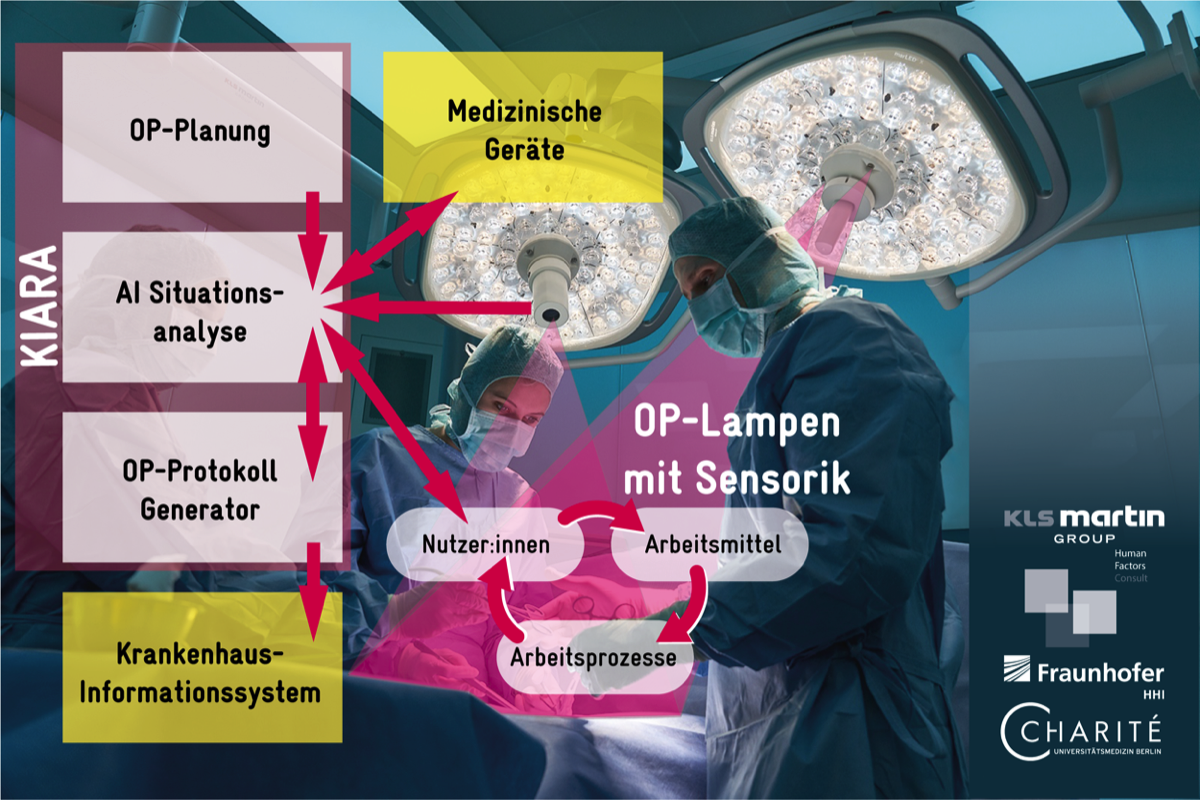
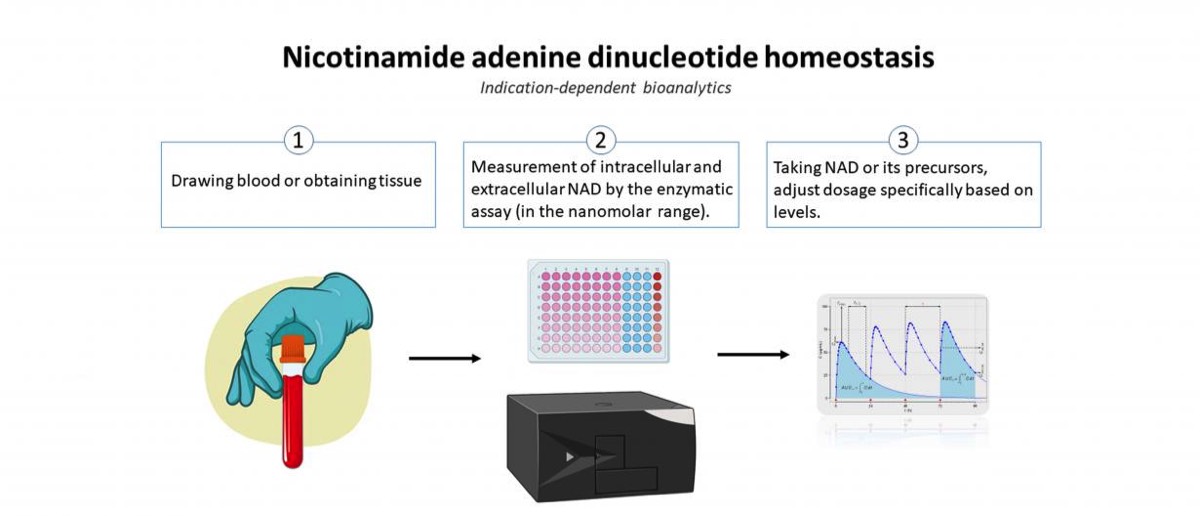
DFG-funded ExTra Trial

Liver transplantation in Germany is severely limited by a critical shortage of acceptable grafts and a high mortality rate on the waiting list. Furthermore, a significant number of organs are declined due to quality concerns. As demonstrated in pilot studies in the UK, Netherlands, Australia, and the United States, declined liver grafts can and should be used for transplantation after quality assessment by normothermic ex vivo liver machine perfusion (NMP).
The ExTra trial is a randomized controlled trial with a specific focus on patients with a model for end-stage liver disease (MELD) score ≤25 that are not eligible for (non)standard MELD exceptions. This cohort of patients faces an unacceptably long wait time for transplantation, which increases their mortality risk while on the waitlist. The ExTra trial aims to demonstrate that the time-to-transplant for these patients is shortened through the use of grafts that were initially declined for transplantation but fulfill specified quality criteria on normothermic ex vivo machine perfusion assessment. A total of 186 patients will be randomized in a 1:1 fashion to the experimental arm, which consists of a 12-month option to receive a liver graft that was declined by all German transplant centers but meets specified quality criteria, in addition to listing for liver transplantation through the standard allocation process. The control arm will consist of patients who are waitlisted for liver transplantation through the standard allocation process. Liver grafts that have been declined for transplantation must meet specific quality criteria. These include a maximum of 60% macrovesicular steatosis, no fibrosis greater than stage F3, and no cirrhosis. In line with previously published viability criteria for initially declined liver grafts, the decision to use or decline the graft will be made at least four hours after the start of perfusion.
The ExTra trial aims to show that by expanding the donor pool to include the ExTra option of non-transplantable organs, which appear to be usable after machine perfusion, patients without a high MELD score can be transplanted significantly faster. The ExTra trial is thus the first study worldwide in which this concept will be investigated in a randomized clinical trial. The study should make an important contribution to expanding the donor pool for liver transplants and thus ultimately help all patients on the waiting list for liver transplantation.