New Book: Virtual participation, real involvement – Transformative technologies for a more inclusive society
Virtual reality (VR) and augmented reality (AR) are among the fastest-growing technologies of the 21st century. They also open up enormous opportunities for social integration: through cultural and educational offerings, through networked digital interaction spaces, or as a means of promoting citizen participation. The contributions in this volume present a wide range of application scenarios for VR and AR in the fields of education, health and public space. They demonstrate in a practical way how society, but also companies, can benefit from expanding their technological skills and taking diversity aspects into account. After all, genuine participation is only possible through a sustainable, transdisciplinary and citizen science approach.
Our book chapter ‘Human-Centred Design of Mixed Reality Applications in Medical Education – GreifbAR’ is now available in open access. Authors are Robert Luzsa, Moritz Queisner, Christopher Remde, Igor Sauer, Nadia Robertini and Susanne Mayr.
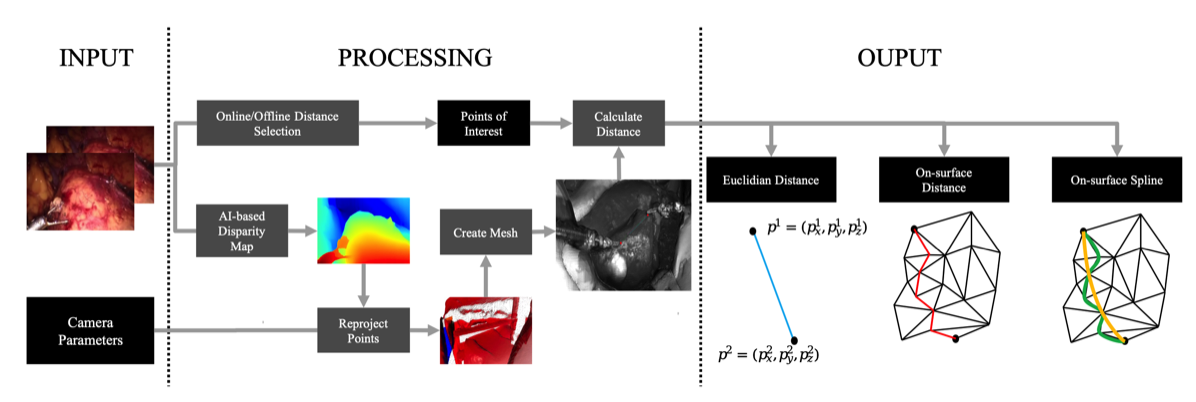
As part of the BMBF-funded project ‘Tangible Reality – Skilful Interaction of User Hands and Fingers with Real Tools in Mixed Reality Worlds’, we investigated how XR technology can be integrated into medical education. The chapter presents an interdisciplinary, XR-based training system for surgical knot tying. It describes key design principles and experiences from development and evaluation. In addition, it proposes a model for the human-centred design of comparable training applications that can also support other projects.
Our book chapter ‘Human-Centred Design of Mixed Reality Applications in Medical Education – GreifbAR’ is now available in open access. Authors are Robert Luzsa, Moritz Queisner, Christopher Remde, Igor Sauer, Nadia Robertini and Susanne Mayr.
As part of the BMBF-funded project ‘Tangible Reality – Skilful Interaction of User Hands and Fingers with Real Tools in Mixed Reality Worlds’, we investigated how XR technology can be integrated into medical education. The chapter presents an interdisciplinary, XR-based training system for surgical knot tying. It describes key design principles and experiences from development and evaluation. In addition, it proposes a model for the human-centred design of comparable training applications that can also support other projects.